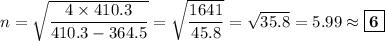Answer:
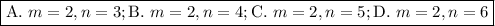
Step-by-step explanation:
The Balmer equation is
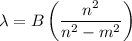
where B = 364.5 nm and m = 2
Thus, the Balmer equation reduces to
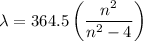
We will be doing four separate calculations for n, so it will be convenient to solve the equation for n.
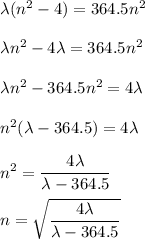
A. λ = 656.5 nm
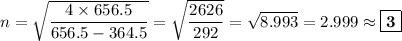
B. λ = 486.3 nm
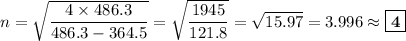
C. λ = 434.2 nm
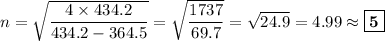
D. λ = 410.3 nm