Answer:
0.60 L.
Step-by-step explanation:
How many moles of
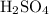 ?
?
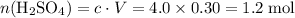 .
.
How many moles of
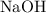 will react with all that 1.2 moles of
will react with all that 1.2 moles of
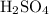 ?
?
Balance the equation:
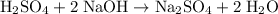 .
.
The coefficient in front of
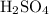 is 1. The coefficient in front of
is 1. The coefficient in front of
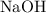 is 2. Hence the ratio:
is 2. Hence the ratio:
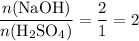 .
.
Therefore
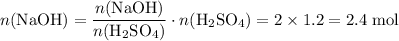 .
.
What will be the volume of the
 solution?
solution?
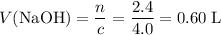 .
.