Answer:
The pH of this solution is 3.4.
Step-by-step explanation:
Consider the definition of the pH of a solution:
![\displaystyle \rm pH = \log{(1)/([H_(3)O^(+)])} = - \log{[H_3O}^(+)]}](https://img.qammunity.org/2020/formulas/chemistry/high-school/r837o9raf2wz474z8zge57vbylygg6vgz8.png) ,
,
where
![\rm [H_3O}^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/4q4kphpnqkph0melg7dpynfg7wtzvhswae.png) is the hydronium ion concentration of the solution in moles per liter. Note that some textbooks write
is the hydronium ion concentration of the solution in moles per liter. Note that some textbooks write
![\rm [H_3O}^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/4q4kphpnqkph0melg7dpynfg7wtzvhswae.png) as
as
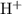 .
.
The unit "M" here is the same as moles per liter.
On a scientific calculator, evaluate
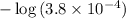
to find the pH of this solution.
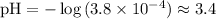 .
.
The pH of this solution is approximately 3.4.