Answer:
2.07 grams
Step-by-step explanation:
We know that the molecular mass of
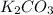 is 138.205 grams.
is 138.205 grams.
In order to find out how many grams we need to make 200 mL having a potassium ion concentration of 0.150, we need to find the number of moles first.
M = moles / liters
0.150 = x / 0.200
x = 0.03 moles
Since there are 2 potassium atoms in each molecule of
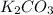 so we will divide this number by half and multiply it by the molecular mass.
so we will divide this number by half and multiply it by the molecular mass.
0.03/2 = 0.015 moles
Mass of
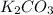 needed = 0.015 × 138.205 = 2.07 grams
needed = 0.015 × 138.205 = 2.07 grams