Answer:
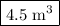
Step-by-step explanation:
Step 1. Calculate the volume of gasoline used.
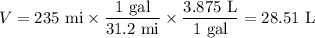
Step 2. Calculate the moles of octane used.
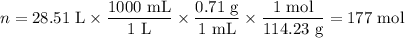
Step 3. Calculate the moles of CO₂ formed
C₈H₈ + 10O₂ ⟶ 8CO₂ + 4H₂O
n/mol: 177
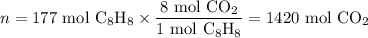
Step 4. Calculate the volume of CO₂
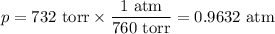
R = 0.082 06 L·atm·K⁻¹mol⁻¹
T = 28 °C = 301.15 K
