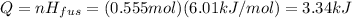Answer:
3.34 kJ
Step-by-step explanation:
FIrst of all, we need to calculate the number of moles corresponding to 10.0g of ice. This is given by
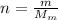
where
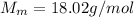 is the molar mas
is the molar mas
m = 10.0 g is the mass
Substituting
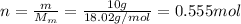
Now we know that the heat of fusion is
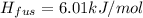
so the thermal energy needed to fuse the ice is