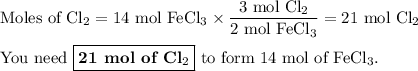Answer:
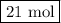
Step-by-step explanation:
(a) Balanced equation
You haven't given the complete reaction, but we can use a partial equation so long as Cl is balanced.
3Cl₂ + … ⟶ 2FeCl₃ + …
(b). Calculation
You want to convert moles of FeCl₃ to moles of Cl₂
The molar ratio is 3 mol Cl₂:2 mol FeCl₃