Answer:
2400 mL
Step-by-step explanation:
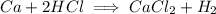
According to this equation, the stoichiometric ratio between
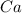 and
and
 for the complete reaction is 1:2.
for the complete reaction is 1:2.
We know that the number of moles of
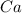 can be calculated using the mole formula. (number of moles = mass / molar mass)
can be calculated using the mole formula. (number of moles = mass / molar mass)
Moles of Calcium =
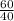 = 1.5 mol
= 1.5 mol
So the moles of
 =
=
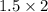 = 3.0 mol
= 3.0 mol
Volume of HCl solution = Moles of HCl/ concentration of HCl
Volume of HCl solution =
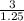 = 2400 mL
= 2400 mL