Answer:
The molarity of the solution remains unchanged.
Step-by-step explanation:
Consider the formula for the molarity
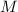 of a solution:
of a solution:
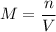 ,
,
where
 is the number of moles of solute in this solution, and
is the number of moles of solute in this solution, and
 is the volume of the solution.
is the volume of the solution.
For this salt solution,
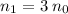 , and
, and
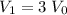 .
.
Initial molarity:
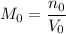 .
.
Final molarity:
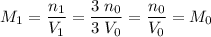 .
.
In other words, the molarity of the solution remains unchanged.