Answer:
There are 0.0186 moles of formula units in 6.35 grams of aluminum sulfate
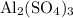 .
.
Step-by-step explanation:
What's the empirical formula of aluminum sulfate?
Sulfate is an anion with a charge of -2 per ion. When sulfate ions are bonded to metals, the compound is likely ionic.
Aluminum is a group III metal. Its ions tend to carry a charge of +3 per ion.
The empirical formula of an ionic compound shall balance the charge on ions with as few ions as possible.
The least common multiple of 2 and 3 is 6. That is:
- Three sulfate ions
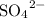 will give a charge of -6.
will give a charge of -6. - Two aluminum ions
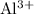 will give a charge of +6.
will give a charge of +6.
Pairing three
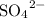 ions with two
ions with two
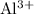 will balance the charge. Hence the empirical formula:
will balance the charge. Hence the empirical formula:
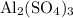 .
.
What's the mass of one mole of aluminum sulfate? In other words, what's the formula mass of
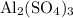 ?
?
Refer to a modern periodic table for relative atomic mass data:
- Al: 26.982;
- S: 32.06;
- O: 15.999.
There are
- two Al,
- three S, and
- twelve O
in one formula unit of
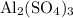 .
.
Hence the formula mass of
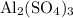 :
:
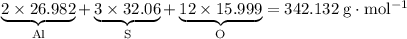 .
.
How many moles of formula units in 6.35 grams of
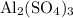 ?
?
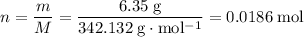 .
.