Answer:
Chemical potential energy converting to heat energy.
Step-by-step explanation:
Burning of wood in a fire place or burning of any other compound in presence of oxygen involves combustion. During combustion there is breaking and making of bonds as combustion is a chemical reaction.
For example:
Combustion of carbon gives carbon dixoide:
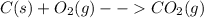
Combustion is an exothermic reaction, it releases large amount of energy.
Thus in all we are converting the chemical energy of a substance to heat energy during combustion.
Hence combustion involves Chemical potential energy converting to heat energy.