Answer:

Step-by-step explanation:
The energy of the incident electron is equal to the energy acquired by the X-rays photon emitted by the tube, which is given by

where
h is the Planck constant
c is the speed of light
 is the wavelength
is the wavelength
In this problem, the wavelength of the photon is

Therefore, the energy is

And keeping in mind that
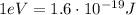
We find the energy in electron volts:
