Answer:
C. 167 kJ
Step-by-step explanation:
The minimum amount of heat require to complete turn the ice into liquid water is equal to the latent heat of the ice, that is, the amount of heat needed by the ice to turn into water. This amount is calculated by:
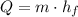 (1)
(1)
Where:
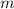 - Mass of ice, measured in kilograms.
- Mass of ice, measured in kilograms.
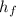 - Latent heat of fusion, measured in kilojoules per kilogram.
- Latent heat of fusion, measured in kilojoules per kilogram.
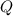 - Latent heat, measured in kilojoules.
- Latent heat, measured in kilojoules.
If we know that
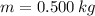 and
and
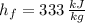 , the latent heat of ice is:
, the latent heat of ice is:
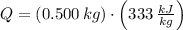
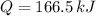
Therefore, the correct answer is C.