Answer:
The energy required to eject photoelectrons from the surface is
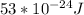
Step-by-step explanation:
Given:
Speed of the photo electron=
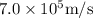
Frequency of the photoelectron=
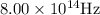
To Find:
The energy required to eject photoelectrons from the surface.
Solution:
Energy of the photon is given by
E = hf
Where,
E = energy of the photons
h = Planck’s constant and its value is
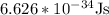
Substituting the values in the formula we get ,
E = hf
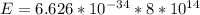

Result:
Thus a energy of
 is required to eject thephotoelectrons from the surface
is required to eject thephotoelectrons from the surface