Step-by-step explanation:
Group 18 is also known as noble gases. Elements of this group are helium, neon, argon, krypton, xenon, and radon.
Period 1 means value of n = 1. Period 2 means value of n = 2. Period 3 means value of n = 3. Period 4 means value of n = 4 and so on, where n is valence shell.
Electronic configuration of helium is
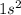 .
.
Electronic configuration of neon is
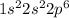 .
.
Electronic configuration of argon is
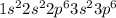 .
.
Electronic configuration of krypton is
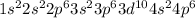 .
.
Electronic configuration of xenon is
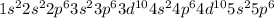 .
.
Electronic configuration of radon is
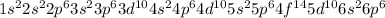 .
.
Therefore, we can conclude that krypton is the atom which is a noble gas in group 18, row 4.