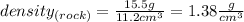Answer:
Explanation:
You need to remember the formula for calculate the density:
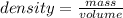
In this case:
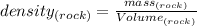
You know that:
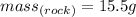
And when it is placed in a graduated cylinder, the volume is displaced from 25.0 mL to 36.2 mL.
Then, the volume of the rock is:
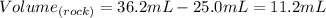
Since
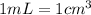 , you can rewrite the volume as:
, you can rewrite the volume as:
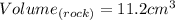
Substituting values into the formula, you get that the density of the rock is: