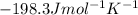Answer: The
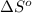 of the reaction is
of the reaction is
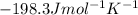
Step-by-step explanation:
Entropy change of the reaction is defined as the difference between the total entropy change of the products and the total entropy change of the reactants.
Mathematically,
![\Delta S_(rxn)=\sum [n* \Delta S^o_(products)]-\sum [n* \Delta S^o_(reactants)]](https://img.qammunity.org/2020/formulas/chemistry/college/od07qddvxcc0son5an5fr21h8boi67g9v0.png)
For the given chemical equation:
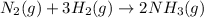
We are given:
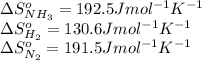
Putting values in above equation, we get:
![\Delta S^o_(rxn)=[(2* \Delta S^o_(NH_3))]-[(1* \Delta S^o_(N_2))+(3* \Delta S^o_(H_2))]](https://img.qammunity.org/2020/formulas/chemistry/college/t7dar7pl6nvkc16jlho6lvyo9g03m0oboa.png)
![\Delta S^o=[(2* 192.5)]-[(1* 191.5)+(3* 130.6)]=-198.3Jmol^(-1)K^(-1)](https://img.qammunity.org/2020/formulas/chemistry/college/5jmbryg3p2s3y56n111gc02vzj26ku9m7l.png)
Hence, the
 of the reaction is
of the reaction is