Answer: The correct answer is Option 2.
Step-by-step explanation:
For the given chemical equation:
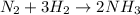
By Stoichiometry of the reaction:
1 molecule of nitrogen is reacted with 3 molecules of hydrogen gas to produce 2 molecules of ammonia molecule.
Hence, the correct answer is Option 2.