Answer:
3.56 %
Step-by-step explanation:
Percent composition of NaCl =
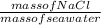 x 100
x 100
Mass of sea water = 520 g
Moles of NaCl = 0.317 mol
Molar mass of NaCl = 58.44 g/mol
Mass of NaCl = moles of NaCl x Molar mass of NaCl
= 0.317 mol x 58.44 g/mol = 18.52 g
Percent composition of NaCl =
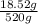 x 100 = 3.56 %
x 100 = 3.56 %