Answer:
\boxed{\text{52.9 kJ/mol}}
Step-by-step explanation:
To solve this problem, we must use the Arrhenius equation:
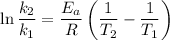
The activation energy depends on the starting temperature, so, let's assume that
T₁ = 25 °C = 298.15 K
T₂ = 35 °C = 308.15 K
k₂/k₁ = 2
This gives
