Answer: Option (D) is the correct answer.
Step-by-step explanation:
A chemical reaction is defined as a reaction that involves exchange of electrons between the combining atoms. Not every chemical reaction releases or absorbs a very large amount of energy per atom.
On the other hand, a reaction that involves change in atomic nuclei of an atom either by splitting or combining of two atomic nuclei is known as a nuclear reaction.
For example,
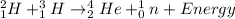
Nuclear fission is a reaction in which a large nucleus splits by absorption of energy into two atomic nuclei. Nuclear fusion is a reaction in which two atomic nuclei combine together to result in the formation of a large atomic nuclei along with release of energy.
Each nuclear reaction releases or absorbs a very large amount of energy per atom.
Therefore, we can conclude that each nuclear reaction releases or absorbs a very large amount of energy per atom.