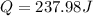Answer:237.98 J
Step-by-step explanation:
The heat (thermal energy) absorbed by the iron skillet can be found using the following equation:
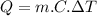 (1)
(1)
Where:
 is the heat
is the heat
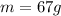 is the mass of the element (iron in this case)
is the mass of the element (iron in this case)
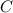 is the specific heat capacity of the material. In the case of iron is
is the specific heat capacity of the material. In the case of iron is
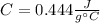
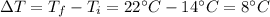 is the variation in temperature
is the variation in temperature
Knowing this, lets rewrite (1) with these values:
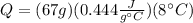 (2)
(2)
Finally: