Answer:
B. Cr in
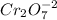
Step-by-step explanation:
Let us first find the oxidation numbers in each of the following scenarios
A. The oxidation number of P in
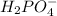 is +5
is +5
because 2H + 4O + P = -1
2(1) +4(-2) + P = -1
P = 5
B. The oxidation number of Cr in
![Cr_(2)O_(7) ]^(-2) \\](https://img.qammunity.org/2020/formulas/chemistry/college/zv67blvzrg3brwv4rzwtfvozsict4v8hfb.png) is
is
2Cr + 7O = -2
2Cr + 7(-2) = -2
Cr = 6
C. The oxidation number of Fe in
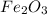 is +3
is +3
2Fe + 3O = 0
2Fe + 3(-2) = 0
Fe = 3
The final answer is Cr