Answer: The oxidation state of chromium in the given compound is +5.
Step-by-step explanation:
Oxidation state is defined as the number which is given to an atom when it looses or gains electron. It is written as a superscript.
If the element gains electron, it will attain a negative oxidation state and if the element looses electrons, it will attain a positive oxidation state.
We are given a chemical compound having chemical formula of
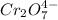
We take the oxidation state of chromium atom be 'x'.
Oxidation state of oxygen atom = -2
Overall charge on chemical compound = -4
Evaluating the oxidation state of chlorine atom:
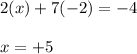
Hence, the oxidation state of chromium in the given compound is +5.