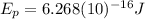Answer:
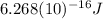
Step-by-step explanation:
The kinetic energy of an electron
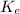 is given by the following equation:
is given by the following equation:
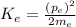 (1)
(1)
Where:
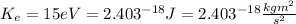
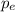 is the momentum of the electron
is the momentum of the electron
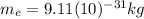 is the mass of the electron
is the mass of the electron
From (1) we can find
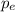 :
:
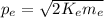 (2)
(2)
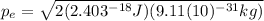
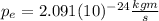 (3)
(3)
Now, in order to find the wavelength of the electron
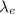 with this given kinetic energy (hence momentum), we will use the De Broglie wavelength equation:
with this given kinetic energy (hence momentum), we will use the De Broglie wavelength equation:
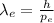 (4)
(4)
Where:
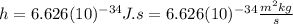 is the Planck constant
is the Planck constant
So, we will use the value of
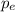 found in (3) for equation (4):
found in (3) for equation (4):
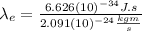
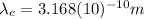 (5)
(5)
We are told the wavelength of the photon
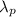 is the same as the wavelength of the electron:
is the same as the wavelength of the electron:
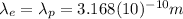 (6)
(6)
Therefore we will use this wavelength to find the energy of the photon
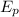 using the following equation:
using the following equation:
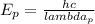 (7)
(7)
Where
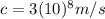 is the spped of light in vacuum
is the spped of light in vacuum
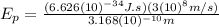
Finally: