Answer:
The pressure increases by a factor 8
Step-by-step explanation:
For a gas held at constant temperature, Boyle's law can be applied. It states that the product of the gas pressure and the gas volume is constant, so we can write:
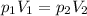
where
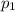 is the initial pressure
is the initial pressure
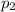 is the final pressure
is the final pressure
 is the initial volume
is the initial volume
 is the final volume
is the final volume
For the gas in this problem, the volume is reduced from
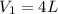
to
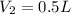
so we can rewrite the equation as
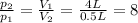
this means that the pressure of the gas will increase by a factor 8.