Answer:
Proton, neutron, electron
Step-by-step explanation:
The atom consists of a nucleus, where almost all the mass is concentrated, and electrons orbiting around the nucleus.
The nucleus consists of two types of particles:
- Proton: it has a mass of
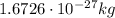 , and a positive electric charge of +e (
, and a positive electric charge of +e (
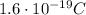 )
)
- Neutron: it has a mass of
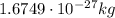 , and it has no electric charge
, and it has no electric charge
The third particle that makes an atom is the electron, that orbit around the nucleus:
- Electron: it has a mass of
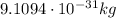 , and it has a negative electric charge of -e (
, and it has a negative electric charge of -e (
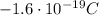 )
)