Answer:
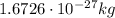
Step-by-step explanation:
The three main particles that make an atom are:
- Proton: its mass is
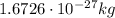 , it carries an electric charge of +e (
, it carries an electric charge of +e (
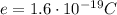 ), and it is located in the nucles of the atom
), and it is located in the nucles of the atom
- Neutron: its mass is
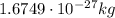 , it carries no electric charge, and it is also located in the nucleus of the atom
, it carries no electric charge, and it is also located in the nucleus of the atom
- Electron: its mass is
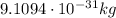 , it carries an electric charge of -e (
, it carries an electric charge of -e (
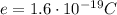 ), and it is located outside the nucleus
), and it is located outside the nucleus