Answer:
Step-by-step explanation:
A hydrocarbon with a double bond in its carbon skeleton is an alkene and has the general form:
-
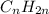 .
.
This is, the number of hydrogen atoms is twice the number of carbon atoms.
On the other hand, alkanes have only single bonds, and the compounds with a triple bond in its carbon skeleton are alkynes.
Review each choice:
1) C₃H₈:
- In this case, the number of hydrogen atoms is 2×3 + 2 = 6 + 2 = 8, which is corresponds to an alkane, not an alkene.
2) C₂H₆
- For this, the number of hydrogen atoms is 2 × 2 + 2 = 4 + 2 = 6. Again an alkane, not alkene.
3) CH₄
- Hydrogen atoms: 1 × 2 + 2 = 4 ⇒ an alkane
4) C₂H₄
- Hydrogen atoms: 2 × 2 = 4. This is precisely the relation for an alkene, so this is the hydrocarbon that has a double bond in its carbon skeleton.
- The chemical formula may be writen as CH₂ = CH₂, to show the double bond.
So, this is the correct answer.
5) C₂H₂
- Hydrogen atoms: 2 × 2 - 2 = 4 - 2 = 2. This relation of carbon and hydrogen atoms corresponds to a compound with triple bond, i.e an alkyne: CH≡CH.