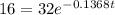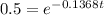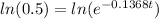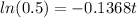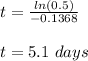Answer:
t=5.1 days
Explanation:
We know that the formula is:
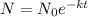
Where N is the amount of substance after a time t,
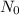 is the initial amount of substance, k is the rate of decrease, t is the time in days.
is the initial amount of substance, k is the rate of decrease, t is the time in days.
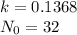
We want to find the average life of the substance in days
The half-life of the substance is the time it takes for half the substance to disintegrate.
Then we equal N to 16 gr and solve the equation for t