Answer:
3.4moles
Step-by-step explanation:
Given parameters:
Number of molecules = 2.04 x 10²⁴
Unknown: Number of moles
Solution
We use the concept of mole to solve this kind of problem.
A mole is the amount of substance that contains Avogadro's number of particle i.e 6.02 x 10²³ molecules
Solving
For elementary particles:
Number of moles =
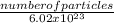 ¹ molecules
¹ molecules
Number of moles =
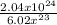
Number of moles of H₂O = 0.34 x 10¹ = 3.4moles