Answer:
The law of conservation of matter (or mass), also known as the Law of Lomonosov-Lavoisier, states the following:
"In a chemical reaction the sum of the mass of the reactants is equal to the sum of the mass of the products."
Hence the famous phrase:
"The mass is not created or destroyed, it only transforms."
This was raised by the Russian scientist Mikhail Lomonosov in 1748 and independently discovered years later by the French chemist Antoine Lavoisier in 1785.
It should be noted that this principle is quite accurate for low-energy chemical reactions, but for nuclear reactions (collisions between particles at high energies), this classical definition does not apply (the total mass of the system does not have to be strictly conserved) and must be taken into account the equivalence between mass and energy that was postulated in Albert Einstein's theory of relativity:
"The amount of mass-energy that manifests a certain space-time is constant throughout the universe."
Being this expressed mathematically by his famous equation where he relates the energy
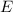 with the mass
with the mass
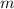 and the speed of light
and the speed of light
 :
: