The empirical and molecular formula : Mg₃Si₂H₂O₈ and Mg₆Si₄H₄O₁₆
Further explanation
Given
28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O
Required
The empirical and molecular formula
Solution
Mol ratio : Mg : Si : H : O =
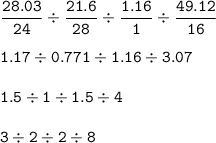
So empirical formula :
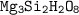
(Emprical formula)n=molecular formula
(Mg₃Si₂H₂O₈)n=520.8
(24.3+28.2+2.1+8.16)n=520.8
(258)n=520.8⇒n=2
The molecular formula : Mg₆Si₄H₄O₁₆