Answer: Option (d) is the correct answer.
Step-by-step explanation:
Since it is given that temperature of hot water is 40 degree celsius. So, when cold water is added into it then it will absorb heat from the hot water and hence, temperature of cold water will become equal to 40 degree celsius.
Therefore, a thermal equilibrium will be maintained as temperature of both hot and cold water becomes equal. In thermal equilibrium, hotter body loses the heat and colder body absorbs the heat.
Therefore, we can conclude that the true statement about the hot water in the calorimeter is its final temperature was
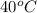 .
.