Answer: Option (B) is the correct answer.
Step-by-step explanation:
According to Le Chatelier's principle, any disturbance causes in an equilibrium reaction will shift the equilibrium in a direction that will oppose the change.
For example,
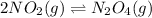
When we increase the temperature then the reaction will shift in a direction where there will be decrease in temperature.
This, means that the reaction will shift in the backward direction.
Thus, we can conclude that if the reaction is at equilibrium and the temperature increases, the equilibrium will shift so that there is more nitrogen dioxide.