Answer:
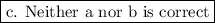
Step-by-step explanation:
A buffer pair consists of either
- A weak acid and its salt or
- A weak base and its salt
(a) Oxalic acid and sodium oxalate
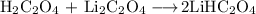
Oxalic acid is an acid and lithium acid is a base. Together, they will neutralise each other and form the salt LiHC₂O₄.
A solution of LiHC₂O₄ is not a buffer.
The correct buffer pair is H₂C₂O₄ and LiHC₂O₄
(b) Carbonic acid and sodium carbonate
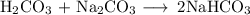
The carbonic acid and the sodium carbonate will neutralise each other and form the salt NaHCO₃.
A solution of NaHCO₃ is not a buffer.
The correct buffer pair is H₂CO₃ and NaHCO₃
