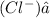Answer: The role of water in the given reaction is that it separates the hydrogen ion
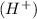 from the chlorine ion
from the chlorine ion
Step-by-step explanation:
Ionization reaction is defined as the reaction in which an ionic compound dissociates into its ions when dissolved in aqueous solution.
HCl is an ionic compound made by the combination of hydrogen and chlorine atoms.
When HCl is dissolved in water, it leads to the ionization of the compound and separates the ions.
The chemical equation for the ionization of HCl follows:
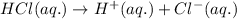
Here, aqueous solution represents the compound is dissolved in water.
Hence, the role of water in the given reaction is that it separates the hydrogen ion
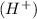 from the chlorine ion
from the chlorine ion