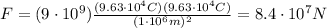Answer:
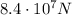
Step-by-step explanation:
The electrostatic force between two objects is given by:
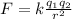
where
k is the Coulomb's constant
q1 and q2 are the charges of the two objects
r is the separation between the two objects
In this problem, we have two boxes separated by

The first box contains
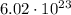 protons, so its charge is:
protons, so its charge is:
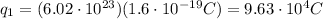
The second box contains
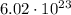 electrons, so its charge is:
electrons, so its charge is:
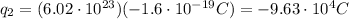
We are only interested in the magnitude of the force, so we can neglect the negative sign and calculate the electrostatic force as: