Answer:
Option B is correct
Step-by-step explanation:
The ideal gas formula can be used to find the Pressure of Nitrogen gas.
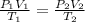
Here P is pressure, V is volume and T is temperature.
In the given question:
P₁ = 1.7 kPa
T₁ = -10°C Changing to Kelvin: 273.15 -10°C = 263.15 K
V₁ = 7.5 m^3
V₂ = 3.8 m^3
T₂ = 200 K
P₂ =?
Putting values in the formula
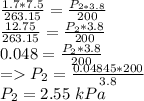
So, Option B is correct.