Answer:
Step-by-step explanation:
Fe + Cl₂ → FeCl₃
a. The balanced equation
All chemical equations obey the law of conservation of matter. To balance this above equation, we either inspect or use mathematical method to obtain a balanced equation.
We put coefficients a, b and c at the back of the compounds as shown below:
aFe + bCl₂ → cFeCl₃
For Fe:
a = c (i)
For Cl:
2b = 3c (ii)
let a = 1, c= 1
Solving for the unknown b, we have:
b =
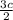
b =
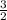
The complete reaction equation is therefore:
Fe +
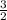 Cl₂ → FeCl₃
Cl₂ → FeCl₃
or
2Fe + 3Cl₂ → 2FeCl₃
Problem a:
Mass of reacting iron = 25g
unknown: number of moles of iron
Solution
To find the number of moles, we apply the mole concept using the expression below:
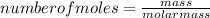
To find the molar mass of the reactant iron, we know atomic mass of iron is 56g.
The molar mass is therefore, 56gmol⁻¹
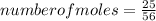
number of moles of the iron reactant = 0.45mole
Problem b:
From the balanced equation of the reaction:
2Fe + 3Cl₂ → 2FeCl₃
2 moles of Fe produces 2 moles of FeCl₃
So 0.45mole of Fe would produce 0.45 mole of FeCl₃
Problem C:
Applying the mole concept;
mass of FeCl₃ = number of moles of FeCl₃ x molar mass of FeCl₃
number of moles of FeCl₃ = 0.45mole
molar mass of FeCl₃ = ?
Atomic mass of Fe = 56g
Cl = 35.5g
Molar mass of FeCl₃ = 56 + (3 x 35.5) = 56 + 106.5 = 162.5gmol⁻¹
mass of FeCl₃ = 0.45 x 162.5 = 73.1g