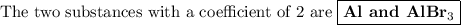Answer:
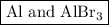
Step-by-step explanation:
Step 1. Pick the most complicated-looking formula (AlBr₃).
Put a 1 in front of it.
HBr + Al ⟶ 1AlBr₃ + H₂
Step 2: Balance Al.
We have fixed 1 Al on the right. We need 1 Al on the left.
Put a 1 in front of Al.
HBr + 1Al ⟶ 1AlBr₃ + H₂
Step 3. Balance Br.
We have fixed 3Br on the right. We need 3Br on the left.
Put a 3 in front of HBr.
3HBr + 1Al ⟶ 1AlBr₃ + H₂
Step 4. Balance H.
We have fixed 3 H on the left. We need 3 H on the right.
We need one more H (½ H₂) on the right.
3HBr + 1Al ⟶ 1AlBr₃ + ³/₂H₂
Oops. Fractional coefficients! We must multiply all coefficients by a number to give integer coefficients.
Step 5. Get rid of fractions.
Multiply all coefficients by2.
6HBr + 2Al ⟶ 2AlBr₃ + 3H₂
All species have a coefficient. The equation is now balanced.