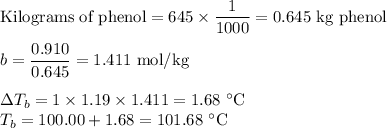Answer:
Step-by-step explanation:
Q8. Boiling point
Data:
m(KOH) = 53.1 g
m(H₂O) = 9.10 kg
K_b = 0.512 °C·kg·mol⁻¹
Calculations:
(a) Moles of KOH
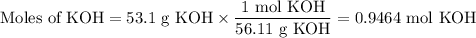
(b) Molal concentration
The formula for molal concentration (b) is
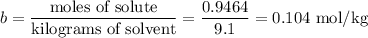
(c) Boiling point elevation
The formula for the boiling point elevation ΔTb is
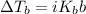
i is the van’t Hoff factor: the number of moles of particles you get from a solute.
For KOH,
KOH(s) ⟶ K⁺(aq) + OH⁻(aq)
1 mol KOH ⟶ 2 mol particles i = 1
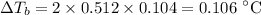
(d) Boiling point
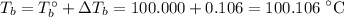
Q9. Freezing point
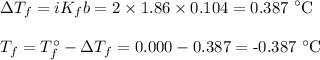
Q10. Boiling point