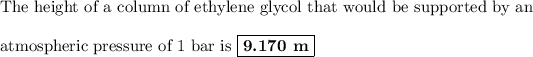Answer:

Step-by-step explanation:
The pressure p exerted by a column of liquid is given by the formula
p = hρg
where
h = height of column
ρ = density of liquid
g = acceleration due to gravity
Data:
p = 1 bar = 100 kPa = 10⁵ N·m⁻² = 10⁵ kg·m⁻¹s⁻²
ρ = 1.112 g·cm⁻³ = 1112 kg·m⁻³
g = 9.807 m·s⁻²
Calculation:
10⁵ = h × 1112 × 9.807
10⁵ = 1.091 × 10⁴ h
h = 9.170 m