Answer:
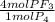
Step-by-step explanation:
Hello
In this case, given the reaction:
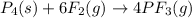
It means that since the coefficients preceding phosphorous and phosphorous trifluoride are 1 and 4, the correct mole ratio should be:
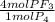
Because given the mass of phosphorous it is convenient to convert it to moles and then cancel it out with the moles on bottom of the mole ratio.
Bes regards!