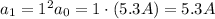Answer:
5.3 A
Step-by-step explanation:
The orbital radius for the generic nth-level in the hydrogen atom is given by
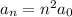
where:
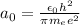
is the Bohr radius, with
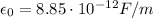 being the vacuum permittivity
being the vacuum permittivity
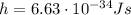 is the Planck constant
is the Planck constant
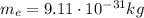 is the electron mass
is the electron mass
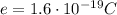 is the electron charge
is the electron charge
Substituting all this numbers into the formula, we find
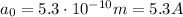
and since
n = 1
the radius of the hydrogen atom for the first principal quantum number is