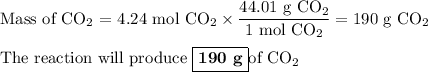Answer:
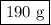
Step-by-step explanation:
We know we will need a balanced equation with masses, moles, and molar masses, so let’s gather all the information in one place.
M_r: 44.01
2C₆H₁₀ + 17O₂ ⟶ 12CO₂ + 10H₂O
n/mol: 6
1. Use the molar ratio of CO₂:O₂ to calculate the moles of CO₂.
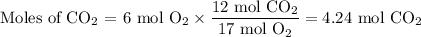
2. Use the molar mass of CO₂ to calculate the mass of CO₂.