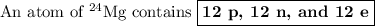Answer:
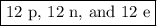
Step-by-step explanation:
No atoms of Mg have a mass of 24.3050 u. That is the average mass of all the isotopes of Mg.
However, the most common isotope of Mg is ₁₂²⁴Mg (mass = 23.99 u)
The atomic number of Mg is 12. It has 12 protons.
The atomic mass of ²⁴Mg is 24. That's the total number of protons and neutrons.
p + n = 24
12 + n = 24
n = 12
An atom of ²⁴Mg has 12 neutrons.
If the atom is neutral, the number of electrons equals the number of protons.
e = p = 12