Answer:
13.35 grams of NH₃ is made if 11 grams of N₂ are used.
Step-by-step explanation:
First of all you should know that the balanced reaction is:
N₂(g) + 3 H₂-> 2 NH₃(g)
Then, it is possible to determine the mass of each compound that reacts or is produced in the reaction by knowing the atomic mass of each element:
Then:
- N₂: 2*14 g/mole= 28 g/mole
- H₂: 2*1 g/mole= 2 g/mole
- NH₃: 14 g/mole+3*1 g/mole= 17 g/mole
By reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) you can see that they react or are obtained in moles:
- N₂: 1 mole
- H₂: 3 moles
- NH₃: 2 moles
Then, by the reaction stoichiometry of the reaction, you can see the amounts of mass that react or are obtained:
- N₂: 1 mole*28 g/mole= 28 g
- H₂: 3 moles*2 g/mole= 6 g
- NH₃: 2 moles*17 g/mole= 34 g
To calculate the amount of NH₃ mass produced if 11 grams of N₂ are used, it is possible to use a rule of three knowing the stoichiometry of the reaction. The rule of three used is the following: if 28 grams of N₂ produce 34 grams of NH₃, 11 grams of N₂ how many grams of NH₃ will they produce?
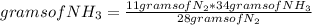
grams of NH₃=13.35
Then, 13.35 grams of NH₃ is made if 11 grams of N₂ are used.