Answer:
A. The enthalpy change for the reaction is 2.825 kJ
Step-by-step explanation:
The given reaction is:
CO + H2O → H2 + CO2
The enthalpy change for a reaction is given as:
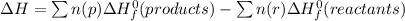
where np and nr are the number of moles of products and reactants
ΔH⁰f are the standard enthalpies of formation of the respective reactants and products
![\Delta H = [1\Delta H_(f)^(0)(H2)+1\Delta H_(f)^(0)(CO2)]-[1\Delta H_(f)^(0)(CO)+1\Delta H_(f)^(0)(H2O)]](https://img.qammunity.org/2020/formulas/chemistry/college/x9lfwa8pkzvrgal21o4i1fwa6i6t5sfcry.png)
Substituting the given enthalpy data:
ΔH = [1(0) + 1(-393.5)] - [1(-110.525) + 1(-285.8)] = 2.825 kJ