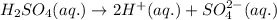Answer:
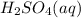
Step-by-step explanation:
An acid is defined as the acid which completely dissociates when dissolved in water and release
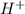 ions in their aqueous states.
ions in their aqueous states.
A base is defined as the base which completely dissociates when dissolved in water and releases
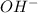 ions in their aqueous states.
ions in their aqueous states.
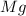 is an element,
is an element,
 is a compound,
is a compound,
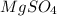 is a salt formed by combination of acid and base.
is a salt formed by combination of acid and base.
The equation for the dissociation of sulphuric acid is given by: